Therapeutic drug monitoring (TDM) means adjusting, controlling and monitoring of drug concentrations in a biological matrix (primarily: whole blood, serum, plasma; but also: cellular components of the blood, saliva, cerebrospinal frior, urine…) for optimization/minimization of the therapeutic and toxic effects, caused by drug therapy.
The official definition of a TDM can be found on the website of the International Society of Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT).
Taking into account the clinical condition of the patient (demographic data, pre-existing conditions or the primary disease, dosages, comedication, times for last intake and sampling) is used to individualise the patient’s pharmacotherapy. In a narrower sense, TDM can help guide decision making to optimize the therapeutic effect and minimize unwanted side effects and supports software-assisted the individual adaptation of drug dosages. A “one-size-fits-all” concept, i.e. amount of drug defined for each patient, regardless of body weight and age, will only be effective for a few pharmaceuticals.
Monitoring of the drug concentration is not required for every drug. Measurement of drug levels is recommended based on national and international guidance documents (Consensus Guideline Neuropsychopharmacology 2017 ; Consensus Report by IATDMCT ; Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology, RiLiBÄK 2023). A TDM service should be carried out in compliance with the indications and under the following conditions:
- insufficient effectiveness
- adverse drug reactions (UAW)
- suspicion of intoxication
- determination of the optimal dosage for prophylactic administration (antiepileptic drugs), or in antibiotic/anti-fungal therapy (avoidance of low concentrations)
- in case of renal and/or hepatic insuffiency (liver, kidney)
- in case of suspicion of disturbed bioavailability
- suspected of lack of compliance, necessity of checking regular intake (adherence)
- Drugs with a strict therapeutic range
- Assessment of the desired therapeutic effect
- large intra- and interindividual variation of absorption and elimination
For the following groups of drugs a therapeutic drug monitoring (TDM) is recommended and offered in a TDM-laboratory:
Antiepileptic drugs: Phenobarbital, Phenytoin, Primidon, Carbamazepine, Oxcarbazepin, Eslicarbazepin, Valproic Acid, Ethosuximid, Lacosamide, Lamotrigine, Levetiracetam, Clonazepam, Clobazam, Topiramat
Neuroleptics: quetiapine, aripiprazole, clozapine, olanzapine, amisulpride
Sedativa: Diazepam, Midazolam, Thiopental, Pentobarbital,
Antidepressants: Sertraline, Citalopram, S-Citalopram, Venlafaxine, Amitriptiline, Imipramin, Trimipramine, Desipramin, Doxepine, Clomipramine
Antiarrhythmics : Amiodaron, DE-Amiodaron
Methylxanthine : Theophyllin, caffeine (in premature and newborn patients)
Antibiotics: Aminoglycosides (Gentamicin, Tobramycin, Amikacin), Vancomycin, Chloramphenicol
Antifungal agents: Posaconazole, Voriconazole, Fluconazole, Itraconazole, Isvuconazole
Immunsuppressants: Mycophenolic acid, Cyclosporin A, Tacrolimus, Sirolimus, Everolimus
However, even in special situations, a TDM of medicines with a high therapeutic width – such as Midazolam in the context of brain death diagnosis – is supposed to be useful.
Antihypertensives: Metoprolol, Amlodipine, Canrenon (Spironolactone), Hydrochlorothiazide
Thyrosine kinase inhibitors: Axitinib, Cabozantinib, Lenvatinib, Midostaurin
……
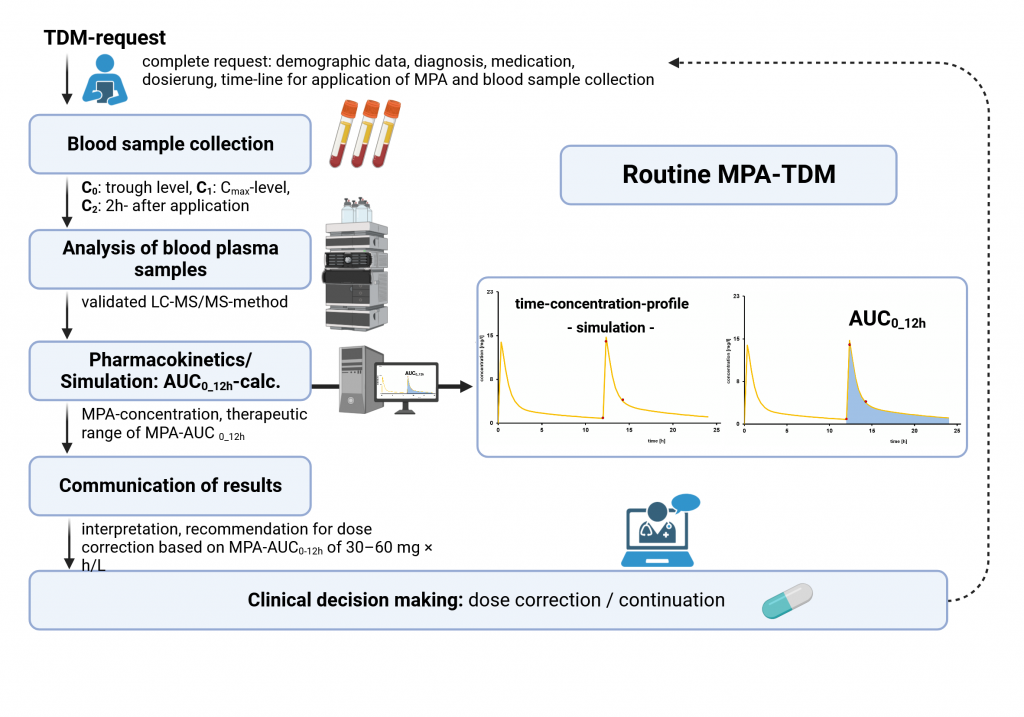
The workflow : from medication intake to clinical recommendation/result