General information:
Substance group: Immune suppressant ATC: L04AA06
Trade names: CellCept®, Mowel®, Mycophenolate mofetil *®, Myfenax®
available dosings: capsules 250 mg, film tablets 250 / 500 mg, powder for the preparation of a
Infusion solution concentrate 500 mg, oral suspension 0.2 g /mL,
Reference ranges:
1.2 – 3.5 mg/L, 0.5 – 5 mg/L (-20/40 mg/L)
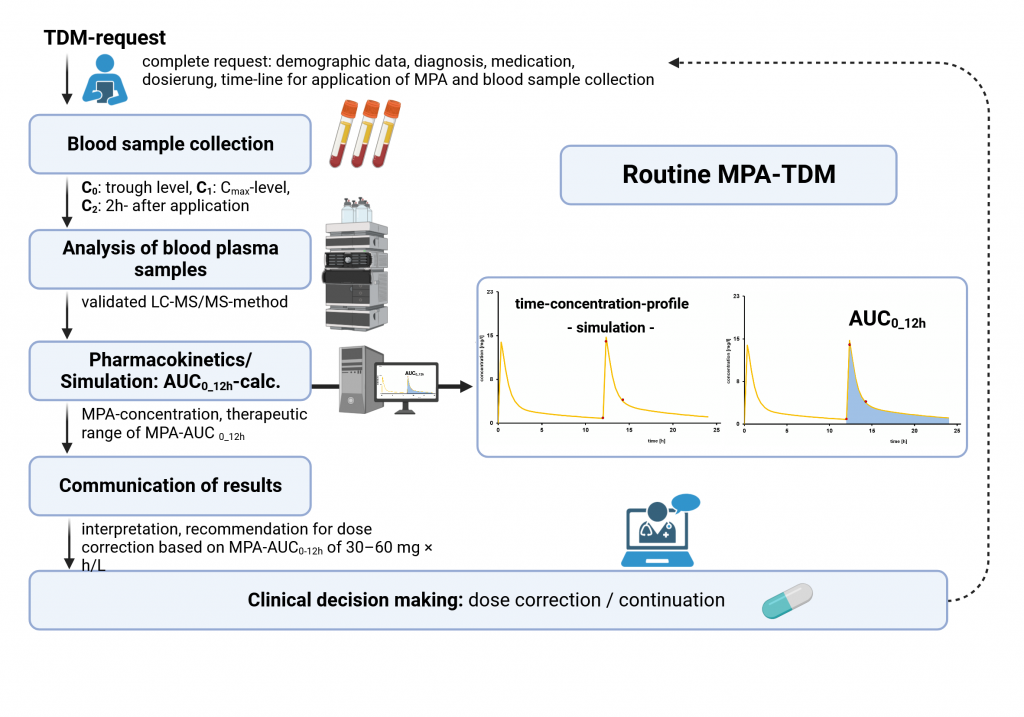
AUC0-12h : 30 – 60 mg/L x h
Toxic plasma level: not applicable
Sampling:
Material: Serum (Monovette brown) Minimum volume for analysis: 50µL Plasma
Acceptance recommendation: To determine the AUC0-12h trough level (immediately before the next intake) and 30 and 120 min afterwards
Analytics:
Test principle: liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS)
Protein precipitation with acetonitrile, chromatographic separation, mass spectrometry
Analysis according to mass charge ratio
Analysis system: triple stage Quadrupol mass spectrometer (LC-MS/MS)
Findings release: usually on the same day
Indication:
TDM recommended:
after organ transplantation, dual immunosuppressive therapy, dose-reduced calcinoine inhibitor therapy, termination or change of calcinoin inhibitor therapy, high immunological risk, delayed or altered organ functions (kidneys, liver, intestines), cystic fibrosis, drug interactions, non-compliance